Quality Concerns Raised Over Intravenous Cannulas in Tamil Nadu Government Hospitals
- bykrish rathore
- 15 November, 2025
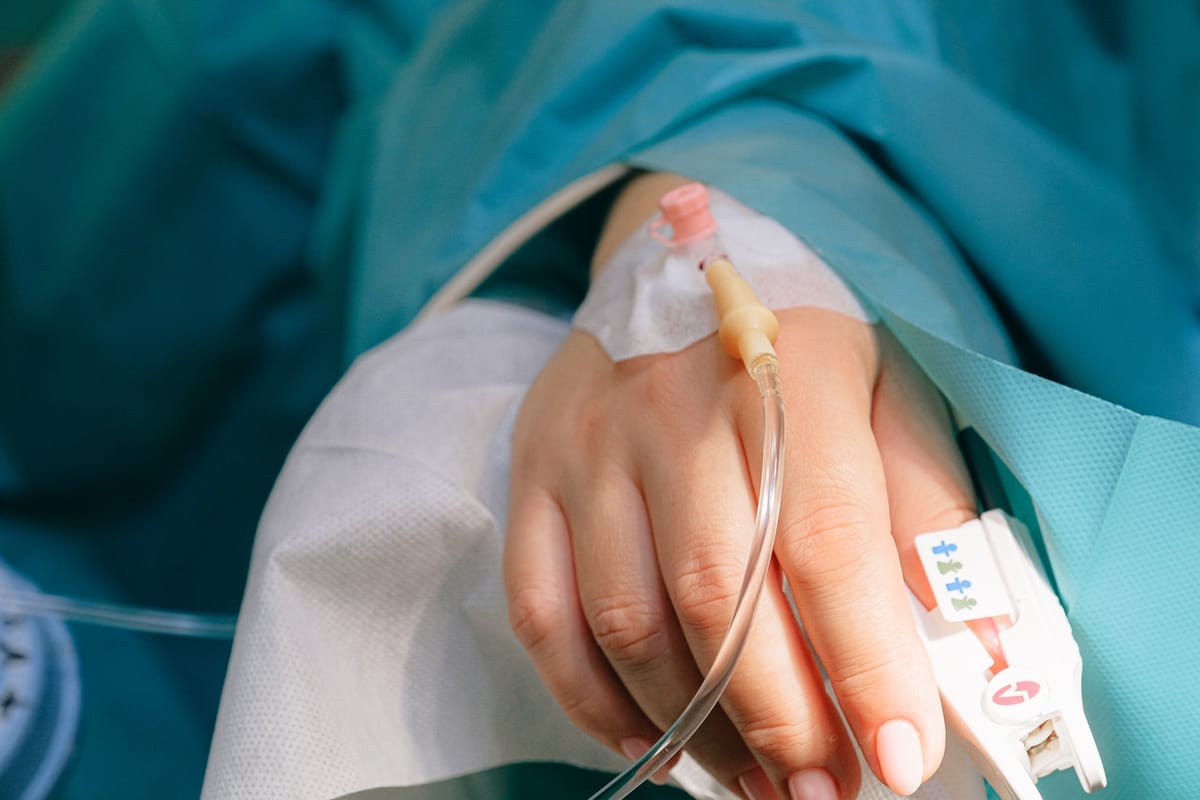
Several government hospitals across Tamil Nadu are reporting alarming quality issues with intravenous (IV) cannulas, sparking concerns about patient safety and the reliability of essential medical supplies. In recent weeks, multiple instances have emerged where cannulas have broken during or after insertion, leaving small plastic fragments inside patients’ veins. These incidents, though isolated to specific batches or suppliers, have triggered widespread discussion about procurement standards, manufacturing quality, and the urgent need for stronger medical safety measures.
Intravenous cannulas are among the most basic and frequently used medical devices in any hospital setting. They are essential for administering fluids, medications, and emergency care. Any defect, however small, can cause significant harm. In the reported cases, the cannula tip or shaft snapped during removal, forcing doctors to conduct additional procedures—even minor surgeries—to retrieve the plastic pieces left inside the bloodstream. Such events not only cause physical pain and stress to patients but also increase the risk of infection, clot formation, inflammation, and long-term vascular complications.
Healthcare workers from several districts have pointed out that the issue may be linked to poor-quality plastic material, weak structural integrity, or faulty manufacturing processes. Some doctors have also suggested that improper storage during transportation or warehouse handling could weaken the cannulas before they reach hospital wards. These concerns have led to calls for an immediate investigation to identify whether the problem lies with a specific vendor, a faulty manufacturing batch, or systemic lapses in monitoring.
The situation has also reignited debate over the procurement system in government hospitals, where bulk purchases often prioritise cost efficiency. Medical professionals argue that essential devices like cannulas should undergo strict quality certification, random sample testing, and independent verification before being distributed across hospitals. While regulatory frameworks exist, gaps in compliance and enforcement can allow substandard equipment to enter the healthcare system.
Patient advocacy groups and public health experts are urging authorities to take swift action, including recalling questionable cannula batches, strengthening vendor vetting procedures, and ensuring transparency in medical supply contracts. They emphasize that even low-cost consumables play a vital role in patient safety and should never be compromised due to budget constraints or administrative oversight.
In response to the emerging reports, hospital administrations in some districts have begun documenting cases, isolating suspect cannula lots, and notifying higher health authorities. The Tamil Nadu Health Department is expected to review procurement records and may launch an inquiry into the involved suppliers. If necessary, the state may also revise its supply chain procedures to ensure better accountability from manufacturers.
Note: Content and images are for informational use only. For any concerns, contact us at info@rajasthaninews.com.
40 के बाद शर्ट से बा...
Related Post
Hot Categories
Recent News
Daily Newsletter
Get all the top stories from Blogs to keep track.





_1772465804.jpg)
_1772465408.jpg)
_1772464394.jpg)
_1772463878.jpg)
