T.N. Government Cancels Sresan Pharma’s Licences, Shuts Down Unit After Cough Syrup Deaths
- bykrish rathore
- 14 October, 2025
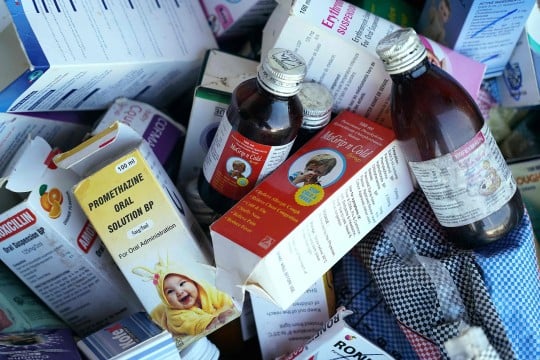
In a decisive regulatory crackdown, the Tamil Nadu government has cancelled the manufacturing licences of Sresan Pharmaceuticals and ordered the company to cease all operations, following the tragic deaths of children linked to its cough syrup, Coldrif.
State inspections discovered that the syrup contained 48.6% diethylene glycol (DEG) — an industrial chemical and known toxin — far beyond permissible limits.
Authorities uncovered over 300 critical and major violations in manufacturing, testing, procurement, packaging, and adherence to good manufacturing / laboratory practices (GMP / GLP).
The company’s owner, G Ranganathan, was arrested by a Special Investigation Team (SIT) from Madhya Pradesh, and has been sent to judicial custody.
This action follows mounting pressure after at least 21–24 children died in Madhya Pradesh after consuming contaminated syrup from the implicated batch.
In addition to cancelling the licence, the Tamil Nadu health and drug control department has ordered comprehensive inspections of all pharmaceutical manufacturing units in the state and suspended two state drug inspectors for dereliction of duty.
This incident has amplified scrutiny on India’s drug regulatory framework, especially concerning quality control lapses, testing failures, and accountability in pharmaceutical supply chains. The World Health Organization (WHO) has also issued a warning concerning contaminated Indian cough syrups.

Note: Content and images are for informational use only. For any concerns, contact us at info@rajasthaninews.com.
40 के बाद शर्ट से बा...
Related Post
Hot Categories
Recent News
Daily Newsletter
Get all the top stories from Blogs to keep track.





_1772465804.jpg)
_1772465408.jpg)
_1772464394.jpg)
_1772463878.jpg)
